What is ISO 13485? – Medical Device Quality Management System
The ISO 9000 quality administration standard framework served as the foundation for the independent QMS standard ISO 13485:2016. The cycle-based paradigm from ISO 9001’s previous iteration, ISO 9000:2008, is modified for the controlled clinical device manufacturing environment in ISO 13485:2016. While ISO 13485:2016 is based on the Plan, Do, Check, Act cycle model principles of ISO 9001, it is designed for administrative consistency; as a result, it is more prescriptive in character and demands an even more thoroughly stated QMS.
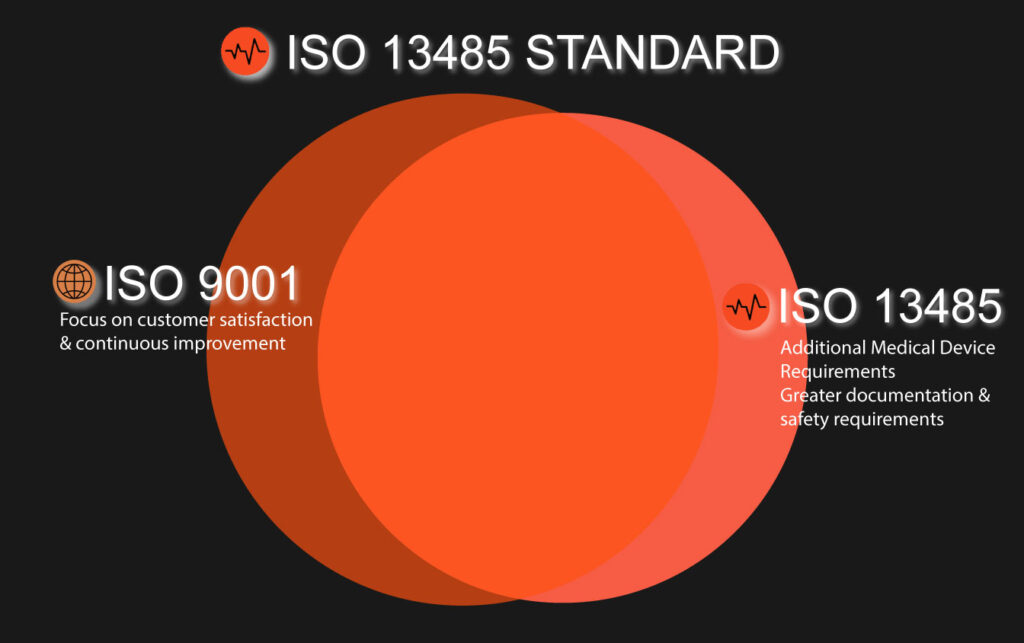
To assist clinical device manufacturers, design a QMS that enhances and maintains the viability of their cycles, ISO 13485:2016 was created. It ensures the predictable design, development, production, installation, and removal of medical devices that are suitable for their intended use.
Such organisations may be involved in at least one stage of the life cycle, such as the planning, development, creation, stockpiling and appropriation, establishment, or modification of a medical device, as well as the planning, development, or organisation of related activities (for example, specialized help). The ISO 13485:2016 standard may also be used by outside parties or providers who provide goods or services to such organisations, such as those related to the quality management framework.
Aside from where it is clearly stated, prerequisites of ISO 13485:2016 are relevant to associations regardless of their size and regardless of their type. Wherever requirements are decided to apply to clinical equipment, the requirements also apply to associated services offered by the organisation.
Although not conducted by the association, the cycles mandated by ISO 13485:2016 that are pertinent to the association are its responsibility and are represented in the association’s quality administration structure by observing, maintaining, and regulating the cycles.
Plan and advancement controls may be excluded from the quality administration framework if pertinent administrative requirements permit their rejection. These administrative requirements may provide optional techniques that need to be attended to in the framework for quality administration. It is the responsibility of the association to ensure that any avoidance of plan and improvement controls is reflected in situations of adjustment to ISO 13485:2016.
ISO 13485 – Medical devices — Quality management systems — Requirements for regulatory purposes

